Mycotoxins & Autism
Fig 1 – 3D rendering of mold with release of its (myco)toxins
Reading time: 5 minutes
Over the last 10 years I have been looking for solutions for patients affected with autism spectrum disorders (ASD). Having explored countless approaches, some good and some not, I feel that we are finally getting somewhere in regards to improving the lives of these individuals. And this sometimes dramatically. I collected, betted and incorporated a lot of known agreed upon research findings about ASD and incorporated them into a looped system that appears to be a well-oil biochemical orchestra feeding on itself. And it’s most often not exactly a symphony.
Since the dramatic increase in autism prevalence over the last decades could be only partially attributed to better diagnosis practices, the possible role of environmental factors triggering the disorder has been proposed. It is now agreed upon that genetic susceptibilities to various epigenetic factors from the environment might be implicated in the increased risk of neurodevelopmental disorders, including autism (ASD) [1]. Heavy metals, pesticides, and other toxins found in food and elsewhere are recognized risk factors for ASD and may contribute to its severity [2].
Here I want to briefly discuss the implications of toxins secreted by mold in autism spectrum disorders and touch upon possible solutions.
Mycotoxins from mold
Mycotoxins are produced by fungi, mainly by the aspergillus, fusarium and penicillium genera, and are not only inhaled in contaminated areas, but also found in our food:
- products containing corn (high fructose corn syrup HFCS),
- peanuts [3],
- wheat, barley and grains (e.g. cereals, legumes),
- baby foods,
- infant formula,
- coffee,
- milk & other dairy products (e.g. yogurt)
- meat,
- spices,
- licorice,
- beer & wine,
- fruit [4-6]
More than 500 mycotoxins have been discovered, with new ones being added to the list regularly [7-9]. They are known to have toxic effects on the immune and neurological systems, generate inflammation and provoke damages to the intestinal barrier (e.g. “leaky gut”) [10-14].
Leaky gut means that larger than normal molecules pass through the gut wall. The term was first used in the late 70s and early 80s, after alcoholic rats (omg!) were shown to “leak” larger than usual molecules directly from the gut into the blood [36]. Animal studies have shown that OTA is immunotoxic, teratogenic, neurotoxic, hepatotoxic, and nephrotoxic. After a single dose of oral ingestion in humans, mycotoxins stick around for as long as 35 days (840h). There are limits of mold toxins permitted in imported foods, so this is a recognized (and tolerated?) contaminant [9].

Fig 2 – 3D Rendering of “leaky gut”, intestinal cross section with tight junctions coming apart
Ochratoxin A (OTA) and Gliotoxin, produced by Aspergillus and Penicillium species, are among the most abundant food and feed contaminants. They are toxic to the liver and kidneys, and they increase intestinal permeability, provoking protein and toxin trafficking through the intestinal wall, depleting glutathione and altering the so-called ‘gut-brain’ axis.
It is well-recognized that the gut bacteria (microbiome) affects the brain’s physiological, behavioral, and cognitive functions, although its precise mechanism has not yet been fully understood. What we do know, however, is that the change in this communication between the gut and brain impairs critical neuropsychiatric functions and has been reported to be implicated in autism [15]. The OTA mycotoxin has been found in higher concentrations in urine and blood [16] in the ASD group. Interestingly, when recalling the sex-bias in ASD, it was found that OTA exerts a male-specific toxicity [17-19].

Fig. 3 – Aspergillus mold under the light microscopic
Children in general are particularly susceptible to the toxic effects of mycotoxins, due to their heightened sensitivity to immunological, nervous, endocrine and neurotoxic effects, as well as their overall exposure, considering their smaller body mass [20]. The maternal immune protection from pregnancy and during weaning are often not enough to support a child’s immature immune system against these toxins, especially when considering that the detoxifying systems in the liver are not completely effective yet and that children are often treated with broad spectrum antibiotics, leading to further GI inflammation.
What can we do?
I’ve used a few of the tests out there and find the accuracy of Great Plains Laboratory MycoTox test, which screens for eleven different mycotoxins, from 11 mold genera (a total of 40 species of mold), in one urine sample. In addition, genetic single nucleotide polymorphisms (SNPs) as well as neurotransmitter imbalances can be tested for in a multi-faceted approach in an attempt to correct deficiencies and other toxicities.
Since mycotoxins are often found to be contaminating dairy and wheat products [see food list above], it is not surprising that casein and gluten containing foods show higher IgG mediated immune responses in the blood of autistic children [16]. The gluten-free (GF) and casein-free diets demonstrate mixed results in the literature, but it has been shown that diets excluding these highly consumed foods improve GI symptoms [21-23]. Most functional and alternative practitioners recommend this as a first line approach. I hear more and more mainstream pediatricians on board with this as well. Avoid dairy. Avoid gluten. It’s not easy, but it’s cheap.
Post-harvest preventative measures in the literature suggest essential oil usage, including cinnamon oil, clove oil, and bay oil, as it has shown to prevent some mycotoxin production [24]. Turmeric oil, eugenol and whole clove in rice grains have been shown to inhibit other mycotoxin production and growth.
The use of microorganisms, including fungi and bacteria, to degrade mycotoxins in feed and food has been widely demonstrated [25]. For example, lactic acid bacteria and probiotics can bind to mold, including the Bifidobacterium and Lactobacillus species [26-28]. Saccharomyces cerevisiae is among the emerging microorganisms binding with certain mold species, e.g. Aspergillus parasiticus [29-30].
The bacteria Enterococcus faecium can detoxify certain mold types. It is interesting when I observe stool tests that patients present with that have this normal floral bacteria usually on the lower side of normal. Is it showing up low because it is working hard to keep mold at bay?
Additionally, substances that bind to mycotoxins can inhibit their absorption into the blood. Examples include cholesterol (some practitioners use cholestyramine or Welchol, which bind cholesterol in the gut), complex indigestible carbohydrates (e.g. inulin) and activated carbon (charcoal) [31].
Chitosan is the structural element in the exoskeleton of crustaceans (such as crabs and shrimp) and cell walls of fungi. It possesses antioxidant, anticancer and other non-toxic properties. The ability of chitosan to control the growth of fungi (and hence the production of mycotoxins) has been demonstrated. It is apparently so effective that it was suggested to be replaced for conventional fungicides [32]. One study suggests using about 1% chitosan and 1% lemon essential oil could prove effective in decreasing mycotoxins [33]. In one study, Chitosan achieved a maximum removal of Ochratoxin A (OTA) at 90%, among being effective against other mycotoxins [34].
Lastly, the antifungal medication itraconazole (e.g. Sporanox) has gained attention after Baker et al successfully treated a 4 year old autistic patient who presented with high mycotoxin levels [35].
Conclusion
It is the micro – environment where this all plays out. Here we can appreciate how ultimately complex the interplay between genes and the environment really is. In ASD, I propose a self-sustaining biochemical imbalance due to genetic susceptibility and epigenetic influences.
Mold > GI inflammation > Leaky Gut > Drained Liver Defenses > Oxidative Stress > Protein/Toxin exposure to plasma > Histamine Surges > Adrenaline (> psychoactive chemicals, e.g. adrenochrome) > Cortisol > Immunosuppression > Mold
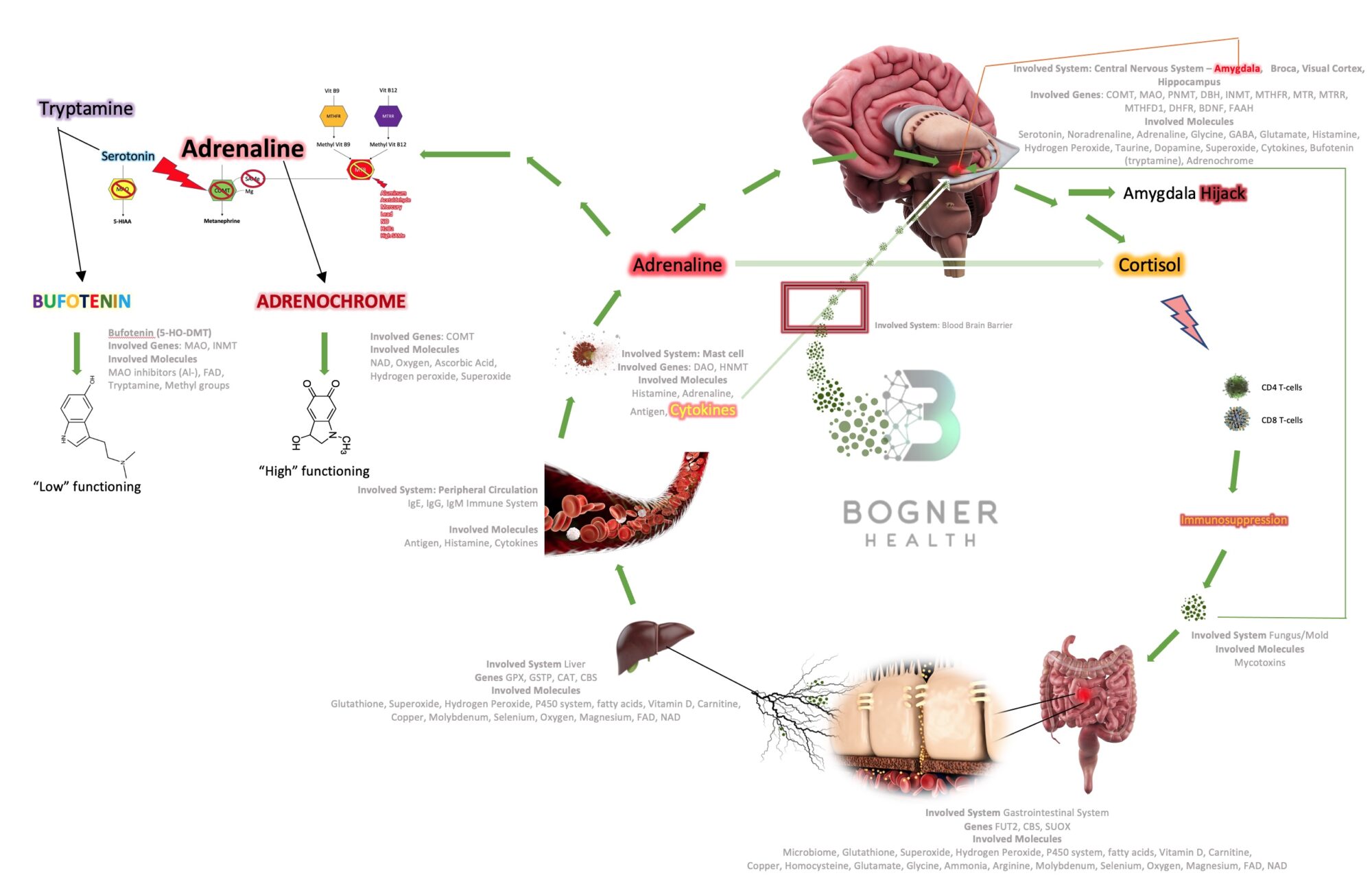
Fig 4 – Mechanisms with correlating genetic and epigenetic influences
Once you plug-in an individual’s tested genetic variation profile into this machine, add about a hundred arrows going back and forth and actually listen to a patient’s complaints, you may appreciate the puzzle pieces coming together. Only then can we truly focus on the sacred individuality of each patient, recognize roadblocks that are necessary to avoid toxicity, correct deficiencies and ultimately bring back the balance our bodies are coded to seek.
We will split up these mechanisms, with more details in regards to well researched genetic variations and environmental influences, in subsequent reports. As always, the best advice I can give is to talk to your primary care provider about this information before implementing any changes to your current regimen.
Best regards,
—
Christian Bogner, MD, FACOG, CFMNP
Bogner Health LLC
References
[1] Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. NeuroToxicology
2008;29(1):190–201.
[2] Kinney DK, Barcha DH, Chayka B, Napoleon S, Munir KM. Environmental risk factors for autism: do they help cause de novo genetic mutations that contribute to the disorder? Med Hypotheses 2010;74(1):102–6. doi:10.1016/j.mehy.2009.07.052.
[3] Bryden, W.L. Mycotoxins in the food chain: Human health implications. Asia Pac. J. Clin. Nutr. 2007, 16, 95–101. [PubMed]
[4] Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [CrossRef] [PubMed]
[5] Bui-Klimke, T.R.; Wu, F. Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [CrossRef] [PubMed]
[6] Bayman, P.; Baker, J.L. Ochratoxins: A global perspective. Mycopathologia 2006, 162, 215–223. [CrossRef]
[7] Urusov, A.E.; Zherdev, A.V.; Petrakova, A.V.; Sadykhov, E.G.; Koroleva, O.V.; Dzantiev, B.B. Rapid Multiple Immunoenzyme Assay of Mycotoxins. Toxins 2015, 7, 238–254. [CrossRef]
[8] Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [CrossRef] [PubMed]
[9] Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [CrossRef]
[10] Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 2004;80:1106–22.
[11] Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis 2010;31(1):71–82. doi:10.1093/carcin/bgp264.
[12] Ohta K, Maekawa M, Katagiri R, Ueta E, Naruse I. Genetic susceptibility in the neural tube defects induced by ochratoxin A in the genetic arhinencephaly mouse, Pdn/Pdn. Congenit Anom (Kyoto) 2006;46(3):144–8.
[13] Paradells S, Rocamonde B, Llinares C, Herranz-Pérez V, Jimenez M, Garcia-Verdugo JM, et al. Neurotoxic effects of ochratoxin A on the subventricular zone of adult mouse brain. J Appl Toxicol 2015;35(7):737–51. doi:10.1002/jat.3061. PubMedPMID:25256750.
[14] Kunio D, Koji U. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci 2011;12:5213–37. doi:10.3390/ijms12085213.
[15] Li Q, Zhou JM. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 2016;324:131–9. doi:10.1016/j.neuroscience.2016.03.013.
[16] Role of mycotoxins in the pathobiology of autism: A first evidence De Santis B;Brera C;Mezzelani A;Soricelli S;Ciceri F;Moretti G;Debegnach F;Bonaglia MC;Villa L;Molteni M;Raggi ME;
[17] Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 2009;76(2): 215–28. doi:10.1124/mol.109.056705.
[18] Mor F, Kilic MA, Ozmen O, Yilmaz M, Eker I, Uran K. The effects of orchidectomy on toxicological responses to dietary ochratoxin A in Wistar rats. Exp Toxicol Pathol 2014;66(5–6): 267–75. doi:10.1016/j.etp.2014.04.002.
[19] Ueta E, Kodama M, Sumino Y, Kurome M, Ohta K, Katagiri R, et al. Gender-dependent differences in the incidence of ochratoxin A-induced neural tube defects in the Pdn/Pdn mouse. Congenit Anom (Kyoto) 2010;50(1):29–39. doi:10.1111/j.1741- 4520.2009.00255.x
[20] Becker-Algeri, T.A.; Castagnaro, D.; De Bortoli, K.; De Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [CrossRef]
[21] Isolauri E, Rautava S, Kalliomäki M. Food allergy in irritable bowel syndrome: new facts and old fallacies. Gut 2004;53(10): 1391–3.
[22] Cai C, Shen J, Zhao D, Qiao Y, Xu A, Jin S, et al. Serological investigation of food specific immunoglobulin G antibodies in patients with inflammatory bowel diseases. PLoS ONE 2014;9 (11):e112154. doi:10.1371/journal.pone.0112154.
[23] Ligaarden SC, Lydersen S, Farup PG. Igg and IgG4 antibodies in subjects with irritable bowel syndrome: a case control study in the general population. BMC Gastroenterol 2012;12:667. doi:10. 1186/1471-230X-12-166.
[24] Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J. Limiting mycotoxins in stored wheat. Food Addit. Contam. Part. A 2010, 27, 644–650. [CrossRef]
[25] Xia, X.; Zhang, Y.; Li, M.; Garba, B.; Zhang, Q.; Wang, Y.; Zhang, H.; Li, P. Isolation and characterization of a Bacillus subtilis strain with aflatoxin B 1 biodegradation capability. Food Control. 2017, 75, 92–98. [CrossRef]
[26] Scott, P.M. Recent research on fumonisins: A review. Food Addit. Contam. Part. A 2012, 29, 242–248. [CrossRef]
[27] Kabak, B.; Var, I. Factors affecting the removal of aflatoxin M1 from food model by Lactobacillus and Bifidobacterium strains. J. Environ. Sci. Heal. Part. B 2008, 43, 617–624. [CrossRef] [PubMed]
[28] Gerbaldo, G.A.; Barberis, C.; Pascual, L.; Dalcero, A.; Barberis, L. Antifungal activity of two Lactobacillus strains with potential probiotic properties. FEMS Microbiol. Lett. 2012, 332, 27–33. [CrossRef]
[29] Giovati, L.; Gallo, A.; Masoero, F.; Cerioli, C.; Ciociola, T.; Conti, S.; Magliani, W.; Polonelli, L. Vaccination of Heifers with Anaflatoxin Improves the Reduction of Aflatoxin B1 Carry Over in Milk of Lactating Dairy Cows. PLoS ONE 2014, 9, e94440. [CrossRef]
[30] Prado, G.; Madeira, J.E.G.C.; Morais, V.A.D.; Oliveira, M.S.; Souza, R.A.; Peluzio, J.M.; Godoy, I.J.; Silva, J.F.M.; Pimenta, R.S. Reduction of Aflatoxin B1 in Stored Peanuts (Arachis hypogaea L.) Using Saccharomyces cerevisiae. J. Food Prot. 2011, 74, 1003–1006. [CrossRef] [PubMed]
[31] Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [CrossRef]
[32] Francesconi, S.; Steiner, B.; Buerstmayr, H.; Lemmens, M.; Sulyok, M.; Balestra, G.M. Chitosan Hydrochloride Decreases Fusarium graminearum Growth and Virulence and Boosts Growth, Development and Systemic Acquired Resistance in Two Durum Wheat Genotypes. Molecules 2020, 25, 4752. [CrossRef]
[33] Gunupuru, L.R.; Patel, J.S.; Sumarah, M.W.; Renaud, J.B.; Mantin, E.G.; Prithiviraj, B. A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduceFusarium head blight and mycotoxin contamination in wheat. PLoS ONE 2019, 14, e0220562. [CrossRef] [PubMed]
[34] Pirouz, A.A.; Selamat, J.; Iqbal, S.Z.; Samsudin, N.I.P. Efficient and Simultaneous Chitosan-Mediated Removal of 11 Mycotoxins from Palm Kernel Cake. Toxins 2020, 12, 115. [CrossRef]
[35] Baker S, Shaw W. Case study: rapid complete recovery from an autism spectrum disorder after treatment of aspergillus with the antifungal drugs itraconazole and sporanox. Integr Med (Encinitas). 2020;19(4):20-27.
[36] The leaky gut of alcoholism. Nutrition Reviews. 2009;43(3):72-74.